of joints and tissues
Think Preservation





Asante Bio is a joint preservation company developing regenerative orthobiologic implants with innovative biomaterials and biomanufacturing technologies
Our biomechanical load-sharing and force-distributing orthopedic devices are being developed to address the biggest unmet needs in orthopedics and sports medicine. We uniquely operate at the intersection of exceptional surgeon experience, biology, and biomechanics.
We are leaders in additive biomanufacturing for regenerative medicine, orthobiologics, and biotextiles.
Asante Bio is developing disruptive tech for high-growth, high-value, high-need orthopedic indications to revolutionize value-based care with our world-class surgeon-developer faculty.
Technical Capabilities
Our Projects
Additive BioManufacturing
We are advancing innovative and proprietary biopolymer biomanufacturing platforms to rapidly produce disruptive and highly differentiated products for demanding surgical jobs. Our platform tech provides unprecedented features for surgical delivery, making for an extraordinary surgeon use experience.
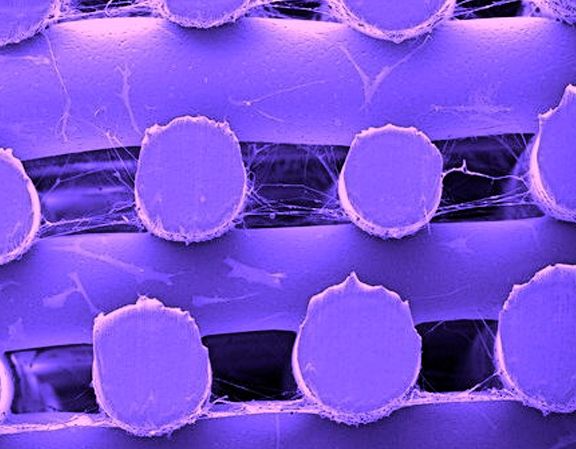
Biomanufacturing is a additive manufacturing production method that uses biological materials, such as biopolymers, extracellular matrices, and cells arranged in 3D to create high-performance implants designed for improving biological healing
Enter the Extracellular Matrix (ECM)
We are additively biomanufacturing natural biopolymers into revolutionary high-strength medical device products with tailored material properties significantly enhanced compared to all competing technologies, made domestically in the US, made economically, and with low environmental impact relative to competing tech.
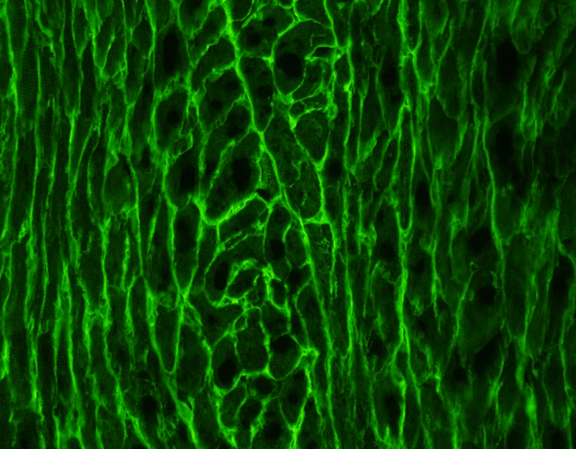
Working with high-performance biological materials, our proprietary additive biomanufactuirng process produces medical device implants with extraordinary material properties to create disruptive orthobiologic, next-generation devices and therapeutics.
Leadership
Our leadership team has expertise in orthopedic surgery, clinical studies, advanced manufacturing and innovative biomaterials and biologics used across myriad indications in regulated markets (BLA, PMA, De Novo, 510(k), and HCT/P), with an extensive track record of successful IP-anchored orthobiologic technology commercialization.
Sponsorships




Message Us!
Interested in learning more or in co-development opportunities? Send us a message.

USA

